Minapharm has established highly competent process RnD and operations teams composed of highly talented Egyptian and international experts. Minapharm uses yeast and mammalian cell line platforms and is also the first African-based biotechnology company to initiate and successfully complete clinical trials for its own biosimilar in Europe.
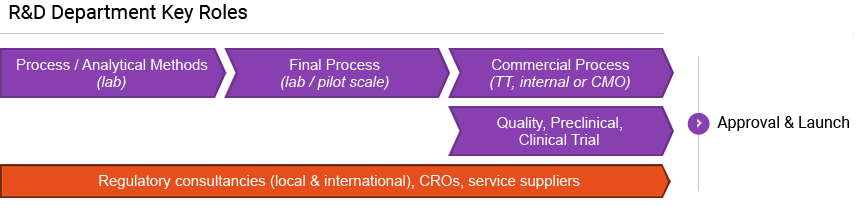

Cell line development

Process development

Local API manufacturing

Finished products
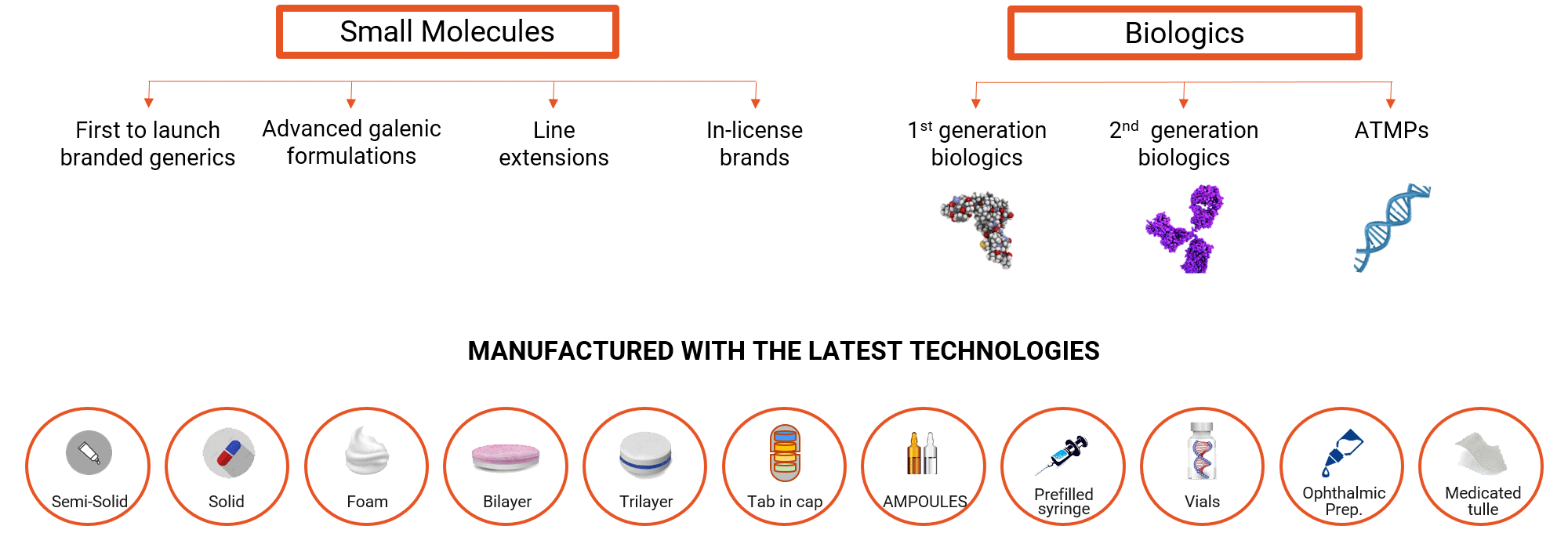
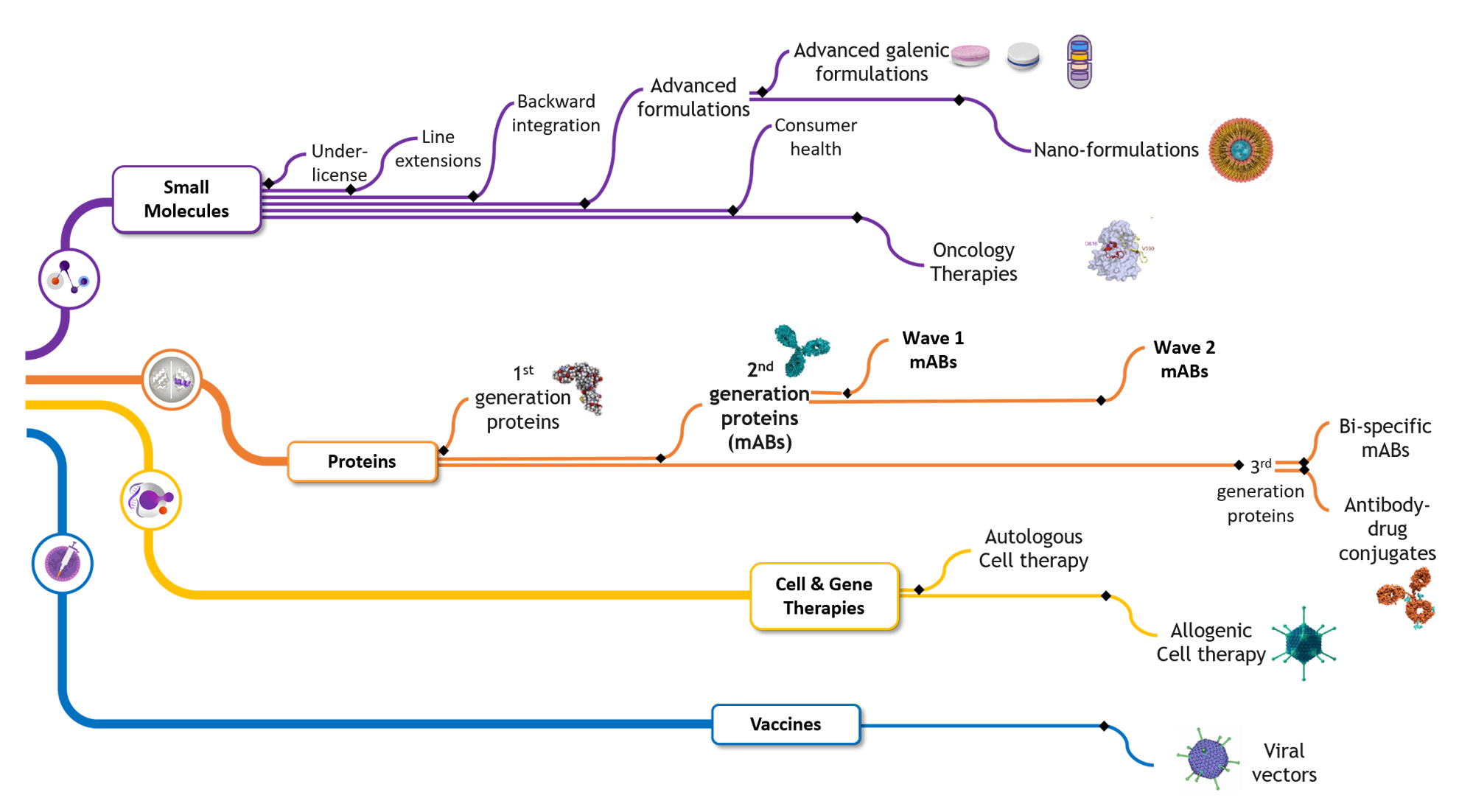
Minapharm develops, manufactures and commercializes over 150 life-saving and life-enhancing products ranging from small molecules to complex genetically engineered self-developed biologics.
Leveraging its Berlin subsidiary, ProBioGen AG, Minapharm offers inventive proprietary technologies to the worldwide biotech sphere. As a globally acclaimed CDMO, ProBioGen specializes in proteins, viral vectors, and cell therapy expertise.
Minapharm developed its own biologics pipeline to ensure quick and efficient processes for the research, trial, manufacture, and delivery of its life-saving and life-enhancing products.
Read about Minapharm’s contributions to the scientific and pharmaceutical community in its industry publications.