Minapharm’s ultimate goal is to strike the balance between innovation and affordability. The
company’s longstanding expertise covers a broad spectrum of medicines, advanced biologics,
and cell and gene therapies. Minapharm relies on a science-driven approach that has already
had a positive impact on the distribution of pharmaceuticals in the Middle East and Africa
as well as globally.
Minapharm’s success is largely the result of its optimized
and self-developed processes. The company’s reliance on end-to-end manufacturing
makes it possible to streamline production and delivery. This translates to great
accessibility of and broader access to the latest pharmaceuticals and
biopharmaceuticals.
Minapharm is a leading pharmaceutical company in Egypt and the Middle East as well as Africa’s largest end-to-end manufacturer and the first biopharmaceutical company in Egypt, Africa, and the Middle East. Headquartered in Cairo, Minapharm has over 25 years of experience in cellular and bioprocess engineering and maintains a portfolio of more than 150 life-saving and life-enhancing products, ranging from small molecules to complex bioengineered proteins.
With its 2000 full-time employees, Minapharm is pursuing its strategy of becoming a global technology provider and manufacturer of high-quality, affordable, and innovative medicines. Minapharm currently exports to more than 15 countries in the Gulf and Africa. Together with its Berlin-based subsidiary, ProBioGen , Minapharm has established an integrated business model, making it the largest biotech company in Africa and the Middle East.
A proud heritage of over half a century of prominent existence in the Egyptian and regional pharmaceutical market, and quarter a century in the global biotech market, to help people around the world with innovative yet affordable medicines.

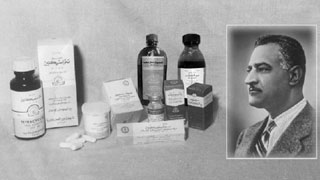
Founder of the company Dr. Saad El Bardissi, during the inauguration ceremony of the first privately owned pharmaceutical company partnering with world leaders in the industry.
Egypt's 1st Private Pharmaceutical Factory

Heliopolis Pharmaceutical laboratories signed the first under-license agreement with Philips-Duphar.
Signing the 1st Under-License Agreement

Pharmaceutical Production of standard and sterile products, in Heliopolis Pharmaceutical factory.
Operation of Heliopolis Pharmaceutical Factory
The economy and industry experienced turbulent times in the history of Egypt, starting from the sixties, merger and nationalization has led to a temporary halt of the production activity.
Heliopolis Factory Nationalization

The ideal link to the pharmaceutical world. MINAPHARM is re-established as a multi-alliance company with worldwide links to the pharmaceutical industry.
Establishing Minapharm Factory and The Beginning of Operations

Minapharm differentiates its portfolio into a specialty pharmaceutical company with dedicated lines in gastroenterology, cardiology, orthopedics and uro-gynaecology.
Minapharm Differentiates Its Therapeutic Portfolio

MINAPHARM enters into a joint-venture with Rhein-Biotech in Germany to establish the first Biotech firm in Egypt, Africa, and the Middle East.
First Joint Venture in Biotechnology in Emerging Markets

Operation of Rhein-Minapharm, uniquely dedicated to the development and manufacturing of therapeutic proteins, using different eukaryotic expression systems (yeast and mammalian cell culture).
Operation of The 1st Biotech Facility in MENA Rhein-Minapharm

Launch of the first IFN for hepatitis C.
Launch of the First IFN for Hepatitis C

Launch of The First PEGylated Interferon
Launch of the First PEGylated Interferon

Launch of recombinant Hirudin portfolio for thrombosis prophylaxis & treatment.
Launch of Recombinant Hirudin Portfolio for Thrombosis Prophylaxis & Treatment
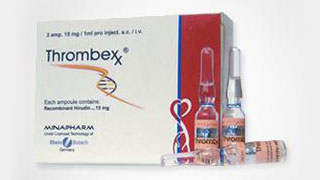
Launch of the world's first topically applied recombinant protein brands: Extrauma and Thrombexx, as well as one of two parenteral forms available worldwide for the treatment of serious thrombotic complications.
Launch of the World's First Topically Applied Recombinant Protein Brands

Expansion of the sterile pharmaceutical area as the state-of-the-art and the most advanced sterile area in Egypt and the Middle East.
Expansion of the Sterile Pharmaceutical Area

Acquisition of ProBioGen: The Berlin-based market leader in cell-line Engineering in Europe.
Acquisition of ProBioGen AG in Berlin
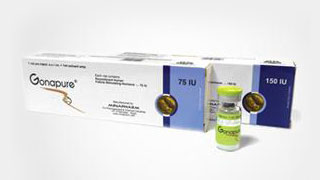
Start of operation of the first Egyptian-Middle Eastern mammalian cell production using genetic engineering. MINAPHARM is launching the first mammalian-derived product Gonapure, recombinant human FSH.
Launching the First Mammalian-Derived Product Gonapure, r- FSH

Expansion of ProBioGen AG (Berlin).
Expansion of ProBioGen AG (Berlin)
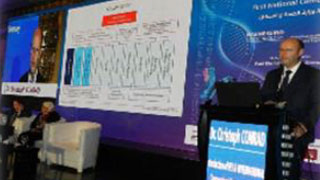
Moderated the 1st national biotech conference with MoH and PEI to release EDA biosimilars guidelines.
Moderating the 1st Egyptian-German Biotech Conference

The 1st African Biologic to Complete Clinical Trials in Germany,r-PTH “Bonosome”.
The 1st African Biologic to Complete Clinical Trials in Germany

Establishment of “MiGenTra” GmbH, for Biosimilars, vaccines, and CGT for Africa.
Establishment of “MiGenTra” GmbH, for Biosimilars, Vaccines, and CGT for Africa
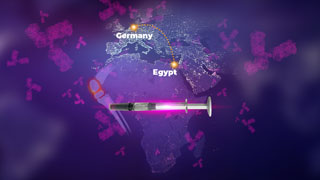
Approval of Phase 1 Multi-center Clinical Trial for Minapharm's Biosimilar Adalimumab in Germany by the Paul-Ehrlich Institute.
Minapharm's Biosimilar Adalimumab is Approved for Clinical Trials in Germany

Launching Teriparatide Biosimilar, Bonosome rDNA.
Launching Teriparatide Biosimilar, Bonosome rDNA
Minapharm is an expert in the field of biopharmaceuticals, and it has three subsidiaries that specialize in different aspects of this field.
Minapharm has established four manufacturing sites, including two expansions in Cairo and Berlin that cater to the development and end-to-end manufacturing of advanced biologics’ therapies.
Minapharm has developed an extensive dynamic network of partners to introduce our products to multiple markets.

Dr. Wafik Bardissi, the Chairman and CEO of Minapharm Pharmaceuticals, has been working in the pharmaceutical and biopharmaceutical industry for more than 25 years. While holding various executive positions, he has become a driving force for pharma and biopharma in Egypt and the Middle East. This includes the 2001 establishment of the first state-of-the-art biotech manufacturing operation in Africa and the MENA region as well as the 2010 acquisition of ProBioGen AG, a global German biotech cellular engineering and CDMO specialist.
Following his studies in Medicine and Surgery, Dr. Wafik pursued his research interests in Business Information Technology (MSc.), Operational Research (MSc.), and Complex Decision Sciences and Artificial Intelligence (PhD) in the UK. In 2010, Dr. Wafik was honored with the Knighthood of the Pontifical Equestrian Order of St. Gregory the Great by Pope Benedict XVI in recognition of his pioneering social work.
Minapharm is committed to provide high-quality, affordable, and innovative medicines to people in need. Our growth and success are only possible with the support of our valued investors.
Learn More